By Patrick Cooney, for AMRC
FEDERAL AGENCY NEWS
FDA Releases Guidance on Modernizing Statistical Methods in Clinical Trials
On January 12, 2026, the U.S. Food and Drug Administration (FDA) issued a draft guidance titled Use of Bayesian Methodology in Clinical Trials of Drugs and Biologics; Draft Guidance for Industry, marking a significant step toward modernizing statistical approaches in clinical research. This guidance is now open for public comment as part of the agency’s commitment under the Prescription Drug User Fee Act (PDUFA) VII to enhance regulatory clarity around innovative trial designs.
According to the press release, the draft guidance is designed to support the use of Bayesian statistical methods in clinical trials across drug and biologics development. Unlike traditional frequentist approaches that rely heavily on fixed hypotheses and p-values, Bayesian methodologies allow scientists to formally incorporate prior information—such as previous study results, real-world evidence (RWE), or external controls—into the design and analysis of clinical studies.
Bayesian approaches can be applied in multiple key aspects of clinical trial design and analysis, including:
- Adaptive decision-making, such as determining futility or success earlier in an ongoing trial.
- Informing dose selection for subsequent trial phases.
- Incorporating external or non-concurrent data sources to supplement limited prospective data.
- Performing subgroup analyses with greater statistical robustness.
- Supporting primary inference—the core statistical conclusion about treatment efficacy and safety.
The agency is accepting public comments, which can shape the final recommendations and influence how these methodologies are implemented in practice. No deadline currently exists for comments.
CAPITOL HILL NEWS
FY26 Funding for HHS Agencies and the FDA
On January 20, 2026, draft text of the Consolidated Appropriations Act for fiscal year 2026 was released. The bill provides the funding and policy framework that will shape federal health programs for the coming year. For AMRC members, the legislation reflects a broader congressional strategy of stabilization rather than expansion—maintaining core research, public health, and health system functions while deferring larger structural reforms to future Congresses.
Congress must act quickly to pass this legislation by the end of January to maintain funding for HHS and its agencies.
The bill sustains federal investment in biomedical research through continued support for the National Institutes of Health (NIH) and other agencies. (See chart below)
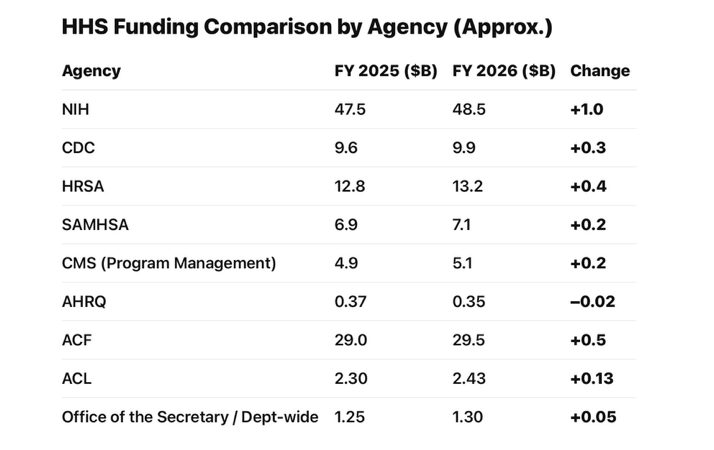
For AMRC members and other MCRCs, this signals that:
- The federal government is prioritizing operational continuity
- Near-term research planning can proceed without major statutory upheaval
- Larger reforms affecting clinical trials and research oversight are more likely to be debated in future authorizing legislation, not annual appropriations bills
Funding for the Food and Drug Administration (FDA) for fiscal year (FY) 2026 is contained in the Agriculture, Rural Development, Food and Drug Administration, and Related Agencies Appropriations Act, 2026 (Public Law 119–37), which was signed into law by President Trump on November 12, 2025. For the clinical research community, the final funding and accompanying policy directives signal a year of operational stability paired with heightened congressional oversight, rather than major regulatory expansion.
For FY 2026, FDA receives approximately $6.96 billion in total resources, including discretionary appropriations and authorized user fees. Congress largely maintained FDA funding at or near prior-year levels, reflecting bipartisan agreement on the importance of sustaining FDA’s core functions—particularly product review, inspections, and compliance—during a period of broader federal budget constraint.
Across multiple provisions, lawmakers reinforced expectations that FDA actions be grounded in updated scientific evidence. Although many of the most explicit riders apply to food and labeling policy, the broader message applies agency-wide: Congress expects FDA to proceed deliberately, particularly where guidance or enforcement could significantly affect regulated stakeholders.
